Field-Cycling Application in Photo-CIDNP

CIDNP (Chemical Induced Dynamic Nuclear Polarization) is a high-sensitivity method for photochemical reactions investigation and radical studies. This manner is also able to apply to protein dynamics investigation, such as protein folding. Studies of CIDNP in weak magnetic fields even give additional information on radical reaction mechanisms and on exchange processes.
Studies of low magnetic field photo-CIDNP require two mechanical breakthroughs, one is a lumination unit, the other is a switcher of magnetic fields. The Lumination unit was developed by using a specific wavelength laser and a light guide to the NMR tube. Nowadays, the lumination unit was alternatively performed by Light-emission diodes (LED) instead of laser lumination. As for the switcher of magnetic fields, one can imply field-cycler to carry the sample to the low-field where the lumination unit is located.
The field-cycling photo-CIDNP combines the fast field-cycling technique and lumination locations at the stray field. The switched external magnetic field CIDNP has been developed already in the 1990s in the Russian group, E. G. Bagruanskay et al, and A. V. Yurkovskaya. The field-cycling setup with mechanical probe transfer and insert of laser/lamp.
Field Cycling Technology Ltd. is currently making the new progress of combining the fast shuttling technique and movable lumination unit along the stray field. The presented photo is a demonstration of lumination in the NMR sample in the shuttling rail. This new technique is in progress and will be planned available in 2022.
Related Literature
Switched external magnetic field (SEMF) CIDNP - a new technique for investigation of the spin dynamics and kinetics of short-lived reaction intermediates in weak magnetic fields
E.G. Bagryanskaya, V.R. Gorelik, R.Z. Sagdeev
Chemical Physics Letters 264 (1997) 655-661
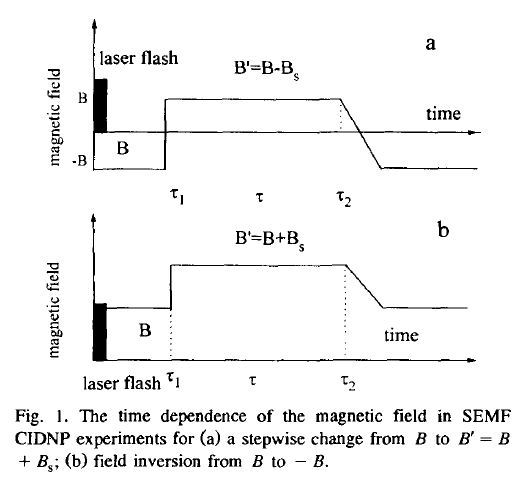
A new time-resolved technique is described to study low magnetic field CIDNP kinetics. The switched external magnetic field (SEMF) CIDNP involves fast switching of a magnetic field during the lifetime of radical intermediates. The information about the spin dynamics and chemical kinetics is obtained from the effect of the magnetic field switch on nuclear polarization of the diamagnetic products of radical reactions. The method has been used to measure the rate constants of a degenerate electron exchange reaction and to provide information on electron polarization in chemical reactions performed in weak magnetic fields.
Field Cycling by Fast NMR Probe Transfer: Design and Application in Field-Dependent CIDNP Experiments
S. Grosset, F. Gubaydullin, H. Scheelken, H.-M. Vieth, and A. V. Yurkovskayal
App. Magn. Reson. 17, 211-225 (1999)
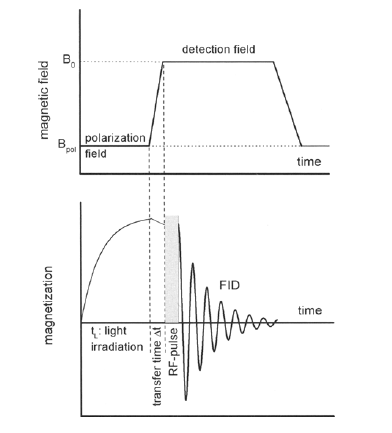
A novel field-cycling unit with fast digital positioning of a high-resolution nuclear magnetic resonance probe in a spatially varying magnetic field is described and used to measure CIDNP spectra of the amino acid-dye (histidine-bipyridyl) photoreaction system in the range between 0 and 7 T. The pattern of nuclear polarization varies with the magnetic field. In particular, strong polarization with an emission/absorption pattern (multiplet effect) is found at low field for two histidine ringprotons with scalar coupling below 3 Hz visible only because of the high resolution made possible by the new field-cycling setup. Also for the CH2 protons in the (3-position a multiplet effect is observed having a pattern changing with magnetic field. By analysis of the spin nutation the non-Boltzmann population differences of the nuclear levels have been determined.
Photo-CIDNP NMR Spectroscopy of Amino Acids and Proteins
Lars T. Kuhn
Topics in Current Chemistry, vol 338. Springer, Berlin, Heidelberg. https://doi.org/10.1007/128_2013_427
Photo-chemically induced dynamic nuclear polarization (CIDNP) is a nuclear magnetic resonance (NMR) phenomenon which, among other things, is exploited to extract information on biomolecular structure via probing solvent-accessibilities of tryptophan (Trp), tyrosine (Tyr), and histidine (His) amino acid side chains both in polypeptides and proteins in solution. The effect, normally triggered by a (laser) light-induced photochemical reaction in situ, yields both positive and/or negative signal enhancements in the resulting NMR spectra which reflect the solvent exposure of these residues both in equilibrium and during structural transformations in "real time". As such, the method can offer - qualitatively and, to a certain extent, quantitatively - residue-specific structural and kinetic information on both the native and, in particular, the non-native states of proteins which, often, is not readily available from more routine NMR techniques. In this review, basic experimental procedures of the photo-CIDNP technique as applied to amino acids and proteins are discussed, recent improvements to the method highlighted, and future perspectives presented. First, the basic principles of the phenomenon based on the theory of the radical pair mechanism (RPM) are outlined. Second, a description of standard photo-CIDNP applications is given and it is shown how the effect can be exploited to extract residue-specific structural information on the conformational space sampled by unfolded or partially folded proteins on their "path" to the natively folded form. Last, recent methodological advances in the field are highlighted, modern applications of photo-CIDNP in the context of biological NMR evaluated, and an outlook into future perspectives of the method is given.